Stress Management is an Important Component of a Weight Loss Strategy
Wednesday,May 7, 2024While stress is a normal part of life, experiencing prolonged periods of stress is not healthy. It’s likely you don’t […]
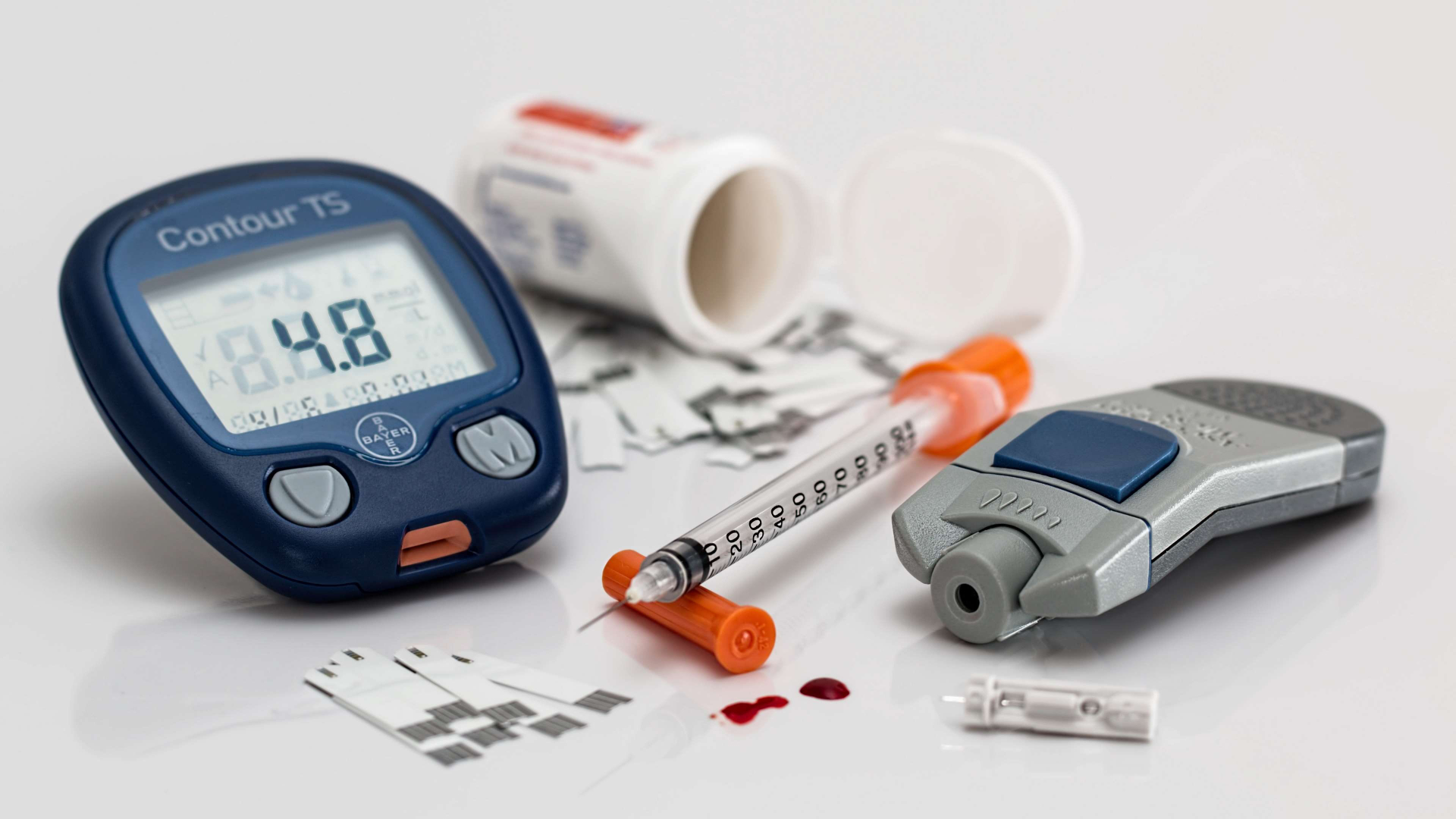
Your Diabetes Guide
Navigate your way through the complex world of diabetes with our guide. Wherever you are on your journey, we can help!

Am I at risk?

What is
diabetes?

How do I
control it?

Learn your
lifestyle

Get
support
High-fat, low-carbohydrate diets may be best for people with diabetes, according to a small Linköping University study. Researchers enlisted 61 […]